Abstract

T-cell acute lymphoblastic leukemia (T-ALL) is an aggressive lymphoid malignancy for which optimal therapeutic approaches for relapsed/refractory disease and biomarkers for upfront risk stratification are currently unknown. Comprehensive, large-cohort bulk-level profiling of T-ALL is currently underway with the promise to reveal novel drivers and associate genetics with treatment response. To synergize with these studies, we designed a single-cell (sc) genomics study powered to comprehensively characterize intra-tumoral arrest states in T-ALL.
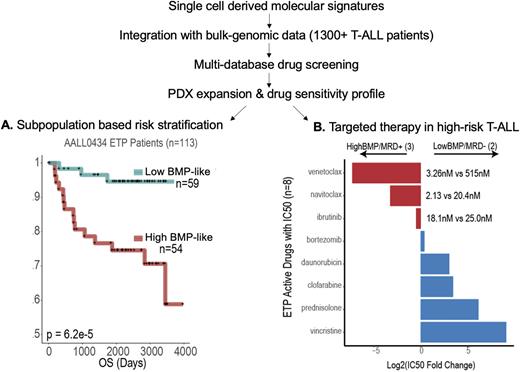
Using integrated scRNA and scATAC-seq analysis on 40 T-ALL cases alongside a novel, single cell atlas of pediatric T-cell development, we identified and characterized a bone-marrow progenitor-like (BMP-like) tumor sub-population associated with resistance to conventional therapy across distinct immunophenotypic subtypes of T-ALL. We first compared 10 treatment-refractory ETP-ALL patients (End of Induction MRD > 20%) to 10 treatment sensitive ETP-ALL patients (EOI MRD negative), where we identified a strong and consistent enrichment of ETP-ALL blasts that were transcriptomically and epigenetically analogous to multipotent BMP. BMP-like blasts had higher surface protein expression of myeloid and stem cell markers, such as CD33, CD123, HLA-DR, and CD34, and lowered expression of T-lineage surface protein markers, such as sCD3, CD4, CD2, and CD10. The top differentially expressed (DE) genes for BMP-like blasts included stem cell markers (C1QTNF4, CD44, LGALS1, HOPX), myeloid-lineage (S100A4, SPINK2), and B-lineage markers (IGLL1, IGKC, IGHM). The top DE transcription factors (TFs) in BMP-like blasts included TFs associated with self-renewal (MEF2C, HOXA3/4/5/6/9/10/11, MEIS1, HHEX, SPI1) and recovery from genotoxic stress (BCL11A). Several of the genes involved in self renewal (MEF2C, HOXA9, FLI1), or T-cell developmental block (SPI1) also had increased motif accessibility. We integrated these signatures with large-cohort bulk-genomic results of 1300+ T-ALL patients treated in the Children's Oncology Group (COG) AALL0434 clinical trial. The molecular signature of BMP-like blasts predicted poor outcome across multiple subtypes of T-ALL (ETP & Non-ETP) and were validated in two independent COG trials (AALL0434, full cohort sequenced; AALL1231, partially sequenced cohort) with corresponding bulk-RNAseq data (Panel A, ETP-ALL patient stratification). We also utilized single-cell derived transcriptional signatures in conjunction with bulk-sample-derived mutation calls to identify fusion drivers (eg, MLLT10, KMT2A, NUP98, NUP214) and gene mutations (eg, NOTCH1 WT, IL7R WT, ETV6 mut, and SAT1B mut) that are associated with the BMP-like state and poor outcome.
Computational analysis and in vitro experiments indicated that BMP-like tumors would be highly resistant to conventional ALL-directed cytotoxics. To address the pressing need for targeted therapy in chemo-refractory T-ALL, we performed multi-database drug screening against the BMP-like signature derived from our single cell data, nominating a number of surface markers (CD44, ITGA4, LGALS1) and intracellular targets (BCL-2, MCL-1, BTK, NFKB) for experimental follow-up in patient derived xenografts (PDX) which we generated and characterized in our single cell study. Notably, experimental studies of PDX expanded blasts indicated consistent and selective vulnerability of BMP-like blasts to apoptosis-inducing agents (Panel B). Other agents with potent activity in this population included TEC-kinase inhibitors and proteasome inhibitors. Follow-up in-vivo studies in PDX are underway with the promise to bridge these therapies to the clinic.
Our work comprehensively defines the developmental arrest states of T-ALL in reference to human early T-cell development. By doing so, we reveal a previously unappreciated sub-population level overlap between patients with distinct subtypes of T-ALL and identify one common BMP-like sub-population that associates with poor response to current therapy. As T-ALL thus far is solely defined by bulk-sample immuno-phenotype, we propose the prospective identification of BMP-like sub-populations and use of non-conventional cytotoxic agents for upfront-risk-stratification and targeted therapy to be realized in high-risk T-ALL.
JX & CC, as well as DTT & KT, contributed equally to the work.
Disclosures
Raetz:BMS: Other: Data and Safety Monitoring Board; Pfizer: Research Funding. Hunger:Servier: Honoraria; Jazz: Honoraria; Amgen: Current equity holder in private company, Honoraria. Yang:Takeda: Research Funding. Mullighan:Pfizer: Research Funding; Abbvie: Research Funding; Amgen: Honoraria; Illumina: Honoraria; Consulting: Honoraria; BEAM: Honoraria; FAZE: Honoraria. Teachey:BEAM Therapeutics: Consultancy; Sobi: Consultancy.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal